By Shim Hyun-tai
Antibody-based therapeutics developer Abclon said Tuesday that it has successfully drawn final candidates for the clinical trials of the next-generation chimeric antigen receptor T (CAR-T) cell therapy for ovarian cancer.
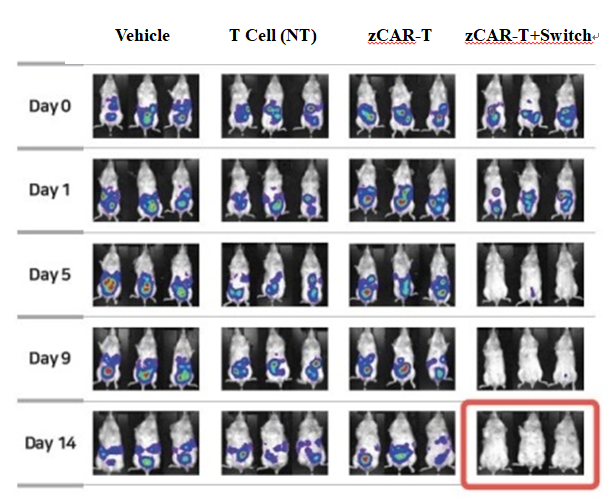
Abclon plans to enter clinical trials in the first half of next year as it confirmed that the new drug candidate, AT501, completely removed cancer cells in an ovarian cancer mouse model with a single dose.
Ovarian cancer is called “silent killer” because the symptoms appear after cancer has been progressed for a long time. If the patients find cancer during the early phase, 85 percent of them can completely recover from cancer. If the cancer cells spread to the abdominal cavity, however, the recovery rate drops down to about 25 percent, which is why treatment for late-stage ovarian cancer patients is needed.
CAR-T therapy genetically modifies T cells in patients and induces them to attack and remove specific cancer cells. The existing CAR-T treatment shows excellent efficacy in treating hematologic malignancy, but it also has an issue of toxicity and no precise results in the field of solid cancer.
Pharmaceuticals around the globe are focusing on developing technologies to find breakthroughs. If Abclon’s AT501 begins clinical trials this year as scheduled, the company expects it will be able to be a leader in the field.
Abclon’s switchable CAR-T platform (zCAR-T) uses cotinine switch molecules to regulate the activity of CAR-T and solve the problems of the same existing type of treatments, which are toxicity, resistance, and disease scalability. AT501 derived from the zCAR-T platform is expected to break through the limitations of the existing therapies.
Abclon said that CAR-T treatments from leading global companies are offering new treatment opportunities for patients with blood cancer, but they are having a hard time treating solid cancer.
“The zCAR-T technology is an innovative platform in the field of CAR-T therapy. We will continue to expand the pipeline of CAR-T therapeutics for solid cancers, including pancreatic and colon cancer, starting with treatments for ovarian cancer, as it is expected to improve safety and increase efficacy,” an Abclon official said.
This article was published by Korea Biomedical Review.