As we step into 2017, a big question looming in the minds of all stakeholders in the cancer research arena is: What is the future of cancer research in the new administration? Members of Congress on both sides of the aisle are supportive of biomedical research, as we witnessed recently with the bipartisan passage of the 21st Century Cures Act by an overwhelming majority. On Dec. 13, President Obama signed the act into law, securing $4.8 billion in federal research funding, including for the Cancer Moonshot and the Precision Medicine Initiative.
Stellar breakthroughs in scientific discoveries, however, need robust, sustained, and predictable annual funding increases for the National Institutes of Health (NIH), and the incoming president’s stance on this issue remains unclear at this time. In this climate of uncertainty, what political and scientific hurdles does the cancer research community face, and what progress can we expect in order to sustain the momentum that we have been experiencing recently in cancer research and treatment?
I asked immunotherapy expert Elizabeth Jaffee, MD, the Dana and Albert “Cubby” Broccoli professor of oncology and professor of pathology at Johns Hopkins University School of Medicine and the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, as well as the associate director of the Bloomberg~Kimmel Institute for Cancer Immunotherapy at Johns Hopkins; precision medicine expert George Demetri, MD, professor of medicine at Harvard Medical School, director of the Ludwig Center at Dana-Farber/Harvard Cancer Center, and director of the Center for Sarcoma and Bone Oncology at Dana-Farber Cancer Institute; and cancer prevention and disparities expert Electra Paskett, PhD, Marion N. Rowley professor of cancer research in the Division of Cancer Prevention and Control at the Ohio State University College of Public Health, to share their thoughts on these aspects.
Although several questions lurk regarding the keenness of the incoming president to support science, the NIH, and the National Cancer Institute (NCI), the experts I spoke to feel we have no reason to fear that progress against cancer will reach a stalemate. The best approach to meeting the challenges that we face today is to stay optimistic, in their view.
Immunotherapy in 2017
“The good news in the field of immunotherapy is that we are learning a lot more about the signals that tumors send to inhibit an effective immune response against them,” says Jaffee, who is a past board member of the American Association for Cancer Research (AACR).
We have already turned this knowledge into therapeutics that inhibit some of these signals (checkpoint inhibitors) so the T cells can be effective in attacking the cancer cells, and developing therapeutics that can activate certain other cells within the tumor microenvironment (checkpoint agonists) to help further activate the T cells, she says.
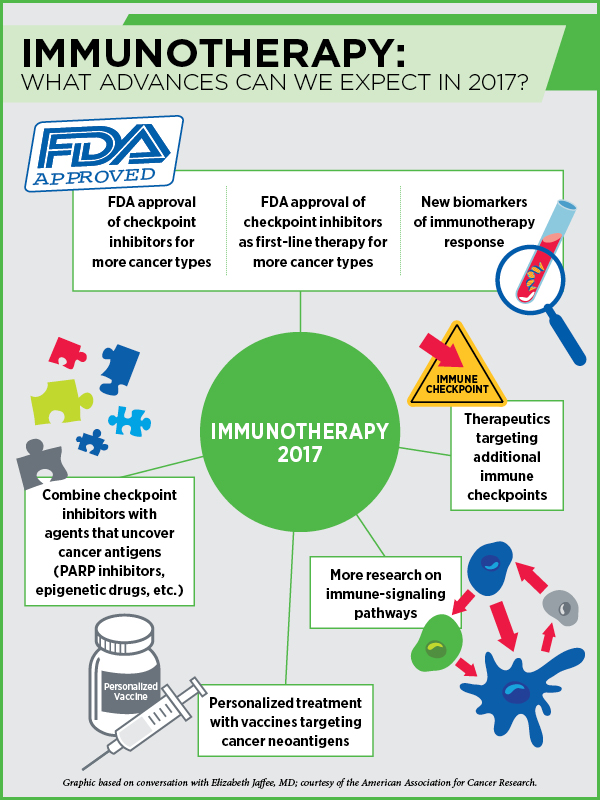
With these approaches, we have been able to convert some metastatic cancer patients with weeks to live into those with chronic disease living a better quality of life, Jaffee adds. In 2017, we are going to see checkpoint inhibitors being approved for more cancer types and as first-line treatment for some cancers, she notes.
The bad news, however, is that these drugs only work for about 20 to 25 percent of all cancers. Further, these drugs can unleash autoimmunity in patients who respond. The side effects can be controlled currently with steroids in some patients, but this year we will learn more about ways to deliver these drugs in a more targeted way to circumvent the toxic side effects, Jaffee says.
“In 2017, I expect to see the development of new drugs that target additional immune checkpoints,” says Jaffee. One reason why almost 70 percent of cancers do not respond to checkpoint inhibitors is that the cancer cells inhibit different pathways that affect T-cell function. Therapeutics targeting immune-evasion mechanisms other than the PD-1/PD-L1 checkpoint, such as IDO, CD40, OX40, TIM-3, LAG-3, and KIR, are already in early clinical development. We will see them progress to clinical testing, alone or in combination with PD-1/PD-L1 drugs, and some of them may be approved or may come close to approval this year, Jaffee predicts.
This year, we will also see a lot of preliminary data identifying new biomarkers of immunotherapy response, according to Jaffee.
Other approaches to get more patients to respond to immunotherapies include activating T cells using vaccines, radiation therapy, or different types of immune-activating chemotherapies, Jaffee says. Combining immune checkpoint inhibitors with agents that can help uncover cancer antigens, such as PARP inhibitors that can make new tumor antigens available to the T cells, or epigenetic agents that can turn on the expression of certain proteins, is another avenue. “We will start seeing results from such studies this year,” Jaffee notes.
We are likely to make more progress this year in personalizing cancer treatment with vaccines, Jaffee predicts. “We are starting to understand the importance of neoantigens for targeting by the immune system,” Jaffee notes. Tumors of many patients who respond to immunotherapy create neoantigens constantly. If we can identify them by sequencing the tumors, we can develop vaccines against them to jump-start the immune system, she says. “We are going to see several clinical trials trying this approach this year.”
“As a member of the Blue Ribbon Panel, one of the 10 areas we identified as at the point of making huge progress is basic research to better understand the mechanisms behind immunotherapy response,” says Jaffee. Answers to questions such as, “What makes a pancreatic cancer that doesn’t respond to immunotherapy different from melanoma that responds to immunotherapy?” or “Why do some tumors that have the biomarker of response not respond while some that do not have the biomarker respond?” or “How to make CAR T-cell therapy work in solid tumors?” can only be found by pursuing more basic science research, she notes.
“We have the technology to find answers to many basic research questions and there is excitement among academia, industry, and federal agencies to work together; however, we need more funding to pursue such important studies,” says Jaffee. While she is concerned about the uncertainty regarding the scientific priorities of the new administration, she is cautiously optimistic.
Precision Medicine in 2017
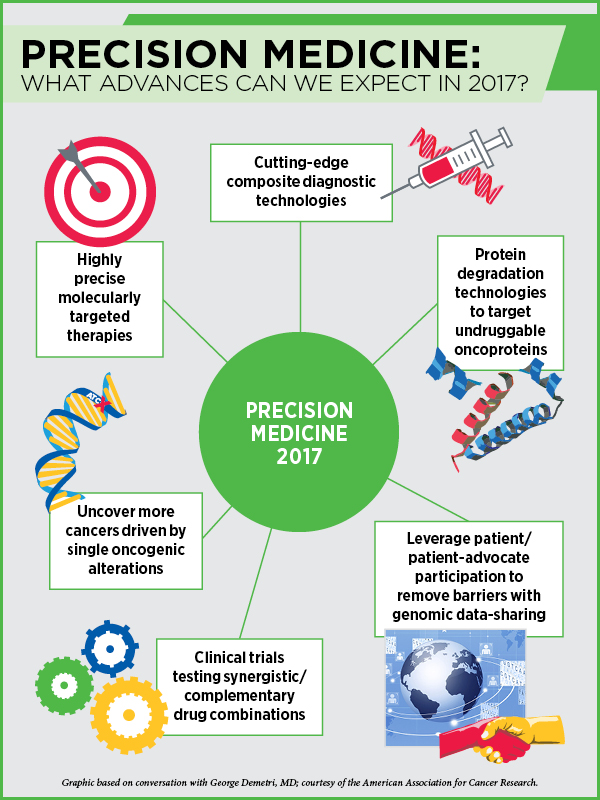
“The good news is that we are still uncovering virtually monogenic diseases – diseases that are driven by single oncogenic fusions or mutations,” says Demetri, also a board member of the AACR. Therapies targeting single mutations, such as NTRK fusions, lead to durable and dramatic responses, he notes.
For the vast majority of common cancers, however, it is not a simple monogenic problem, which would need more combination therapies and more research to find where the Achilles’ heel is, Demetri says. “This is where we, as professionals, need to be careful about overstating the value of precision targeting to the public without also getting too negative.”
“Cancer diagnostics are going to get better and better,” says Demetri, and predicts that we may be on the verge of putting together a composite set of predictive and prognostic biomarkers. “Our diagnostic tools are getting so sophisticated that we can put cancers into different bins at different times in a patient’s treatment course.” With treatment, cancers acquire new mutations to thrive, and with technological advances we can now, in many cases, track the different mutations that are likely driving the disease and match them with different drugs.
While Demetri notes that we have to be intellectually honest about the fact that most patients treated with targeted therapies develop resistance, he is not yet giving up on our aim of finding cures. It may appear as not achievable now, but we are getting there through combination therapies, he adds.
We really need to hone our ability to pick combinations that are not cross-resistant and truly synergistic or complementary, he notes. One of the many approaches is to tie targeted therapies with less-targeted, more multifunctional modalities such as immuno-oncology, which can trigger the immune system. “Even though checkpoint inhibitors are a multibillion dollar market, I would say they are still in their infancy as far as our extent of understanding goes,” notes Demetri.
We are likely to see more efforts in developing very potent epigenetic drugs, according to Demetri. Drugs that target EZH1, EZH2, and bromodomain inhibitors are an alternative way to addressing the bad wiring in cancer cells, notes Demetri, who expects to see more studies testing combinations of epigenetic therapies with targeted therapies and immunotherapies.
This year, Demetri predicts, we will gain further understanding into the smaller, molecularly defined subsets of cancer, and develop even better, more precisely targeted therapies.
A recent development is the work on protein degradation technologies, which make it possible to bring the ubiquitin-proteasome system to degrade a protein of interest in a very catalytic way, Demetri says. “This could be a real game changer,” he predicts. Researchers are still trying to understand how to use the protein degradation system appropriately to target undruggable proteins such as Myc and Ras. “I think this is an exciting area of research and this year we are likely to see some proof-of-concept studies. I feel it is just about to hit the mainstream,” Demetri notes.
Progress with cancer genomic medicine depends on a key element, data sharing—large genomic datasets made available to all so the significance of the genetic alterations present in patients’ tumors could be gleaned through collective evidence. However, data sharing comes with many challenges, such as protection of patients’ privacy and ownership of the data.
Progress with breaking the barriers of genomic data sharing will come from continued advocacy from the patients rather than the professionals, Demetri says. “It is vital to leverage our interactions with patients and patient advocates who want the same things that we do to push the kind of data sharing that will advance the field.”
In the event that the efforts from the federal government to further data-sharing initiatives are insufficient, we may see the private sector jumping in to build databases, he notes. “A lot of this will depend on the next heads of the NIH and the NCI,” he adds.
Regarding the provisions in the 21st Century Cures Act to roll back FDA regulations to accelerate drug development, “I like the idea that we can streamline and simplify and have more transparency in the rules for therapeutic development in cancer, “ Demetri says. “I’m optimistic that we will keep the focus on both safety and efficacy.” Ultimately, if a drug does not work sufficiently well to justify its use or if it is prohibitively expensive compared to other equally effective options, Demetri says he would trust our community of professionals would have the integrity to not use it, and to explain these choices and options to our patients.
We are in the post-genomic era where we need to overlay other elements (such as epigenetic compensatory mechanisms, metabolic or anatomic resistance mechanisms, etc.) on top of simple genotyping and basic interpretation of the genotype, Demetri says, adding this is more complicated and will take a lot of effort and money to fund the necessary research. “I feel that the advances against cancer are very powerful and will go forward no matter what, but the question is, how fast can we get there and at what scale and scope?” Demetri asks.
“Reading the tea leaves of the new administration, we may be facing less than optimal federal support for cancer research, but I’m hopeful that we will see more private sector-based and philanthropy-based partnerships that will step up to support cancer researchers at this important juncture,” Demetri says.
“What I’d like to see this year is some time for positive reflection to realize the importance of public funding of science, tied to the importance of funding investigators who can follow their instincts to make new discoveries,” says Demetri. “Fundamental, curiosity-driven research is the only way we are going to get to unthinkable breakthroughs – real paradigm shifters akin to kinase inhibitors in the late 90s and immuno-oncology drugs more recently.
“We need to recognize that there is a social good to funding basic research – without fundamental scientific understanding, applied medical research is limited to moving already existing therapies around the chessboard. We need science-based novel agents and new approaches to change the therapeutic approach and help patients in ways we might not be able to conceive of today,” Demetri notes. “We have not told that story well enough, and I’d like 2017 to be the year where we make that a little clearer to the public so there’s more support for basic science. That is critical.”
To read this entire article in The Clearity Portal, please click here.