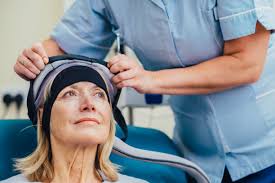
The FDA granted expanded clearance to the Paxman Scalp Cooling System, allowing the device to be used by patients with any type of solid tumor.
The Paxman Scalp Cooling System (Paxman) — designed to reduce hair loss among patients who undergo chemotherapy — received clearance last year for use among patients with breast cancer.
“Scalp cooling has been a real game changer for so many of our patients with breast cancer, minimizing the risk of one of the most dreaded side effects of chemotherapy,” Steven Jay Isakoff, MD, PhD, medical oncologist at Massachusetts General Hospital Cancer Center, said in a press release. “Thanks to the recent expanded FDA indication for the Paxman Scalp Cooling system, so many more patients with solid tumors in the US can now consider this option as a safe and effective way to keep their hair during chemotherapy. We are already working on plans to make this available to all of our patients with solid tumors.”
The Paxman system reduces the temperature of the scalp by a few degrees immediately before, during and after the administration of chemotherapy.
The device is intended to reduce the amount of chemotherapy that reaches the hair follicles, reduce drug diffusion through the cell membrane, decrease cell division, reduce active transport mechanisms and generally reduce metabolic activity.
Paxman has installed approximately 225 scalp cooling systems in the United States since its initial FDA clearance last spring. Another 65 systems are awaiting delivery and installation.
“This is another step forward in making cancer therapy more personalized and putting the patients in the driver’s seat as we create more options and pathways our patients,” Debu Tripathy, MD, professor in and chairman of the department of breast medical oncology at The University of Texas MD Anderson Cancer Center, said in the release.
To read this full article by Healio in The Clearity Portal, please click here to login.