By Gordon Parry, PhD & Cory Bentley, PhD
The human immune system is very powerful. Successfully harnessing the immune system to destroy tumors, cancer immunotherapy, has been a dream of many oncologists. Cancer immunotherapy has become a reality with sipuleucel-T (Provenge) for prostate cancer, ipilumumab (Yervoy) for melanoma, and adaptive T cell therapy in treating some leukemias and lymphomas.
Recent immunotherapy approaches for ovarian cancer are showing more promise than in the past. These approaches include (1) vaccines, (2) treatment with the patient’s own immune cells – adaptive T-cell transfer, and (3) immune check point inhibitors, drugs that remove a brake on the immune system that may be protecting tumor cells. Combinations of new vaccine strategies with chemotherapy and/or adaptive T-cell transfer approaches have made the furthest inroads against ovarian cancer and are the focus of this article. They are being evaluated in women who are newly diagnosed (as maintenance after front line treatment) as well as in women who have recurrent cancer.
A vaccine is a treatment that “teaches” the immune system what the enemy looks like and hence what to fight against. For example, the familiar flu vaccine exposes the recipient to something that looks very much like the flu. Once exposed to the flu vaccine, the body knows what flu looks like and prepares an arsenal of weapons to attack anything that closely resembles what was in the vaccine. Vaccine therapies against cancer also “teach” the immune system to recognize the tumor as an invader. Once educated, the body can mount an attack against the tumor. Some tumor vaccines are made from proteins expressed primarily on tumors, known as tumor-specific antigens. Vaccines created from tumor-specific antigens are known as protein or peptide vaccines. Other vaccines are made from the patient’s own tumor collected at surgery, a completely personalized (autologous) vaccine.
Combination of a Protein Vaccine With Chemotherapy
Promising results from a Phase 1 ovarian cancer trial using a protein vaccine were published in the January edition of Cancer Immunology Research. The tumor-specific antigen, or protein, used to create the vaccineis NY-ESO-1. This study showed the potential of a protein vaccine approach combined with chemotherapy (doxorubicin) and a drug (decitabine) to improve the visibility of the cancer cells to the newly educated immune cells. Dr. Kunle Odunsi of the Roswell Park Cancer Institute, the principle investigator of this study, pointed out that although the purpose of this phase I trial was to assess safety, clinical benefit was seen in up to 60 percent of patients with chemotherapy resistant tumors.
Personalized Vaccine
There are currently 22 actively recruiting “vaccine ovarian cancer” trials listed at ClinicalTrials.gov. Nine of these are Phase 2 trials. The FANG(TM) personalized ovarian tumor vaccine is among the tumor cell vaccines that is furthest along in the development process, having already been tested in Phase 1 and 2 trials with Phase 3 trials planned. (Click here to review a previous newsletter to learn more about the differences between Phase 1, 2, and 3 trials) This vaccine utilizes the patient’s own tumor cells, which are modified in the laboratory to express an immune-stimulatory molecule (GM-CSF) and to block expression of an immune-inhibitory molecule (TGF-b). Recently released results from a randomized phase 2 study (NCT01309230) in high risk stage III/IV ovarian cancer patients presented at the American Society for Gene Therapy meeting, May 2013, demonstrated promising outcomes in the patients treated to date. In the interim study analysis, patients who received the vaccine plus standard of care chemotherapy took more than twice as long for the cancer to progress (470 days; 10 patients) as those who received only chemotherapy (193 days; 5 patients). While promising, the number of patients in this analysis is small and these effects need to be demonstrated in a larger number of women so we will be on the lookout for the final analysis of this Phase 2 study.
Combination of Personalized Vaccines and Adaptive T-cell Transfer
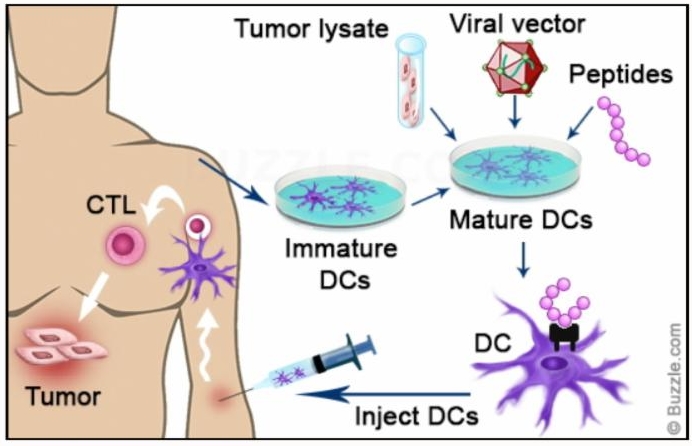
Another approach to personalized vaccine production is to use the patient’s blood AND tumor cells to create a vaccine. Key cells for triggering an immune response, called dendritic cells, are isolated from the blood and then exposed to an extract from the tumor cells in the lab.
Exposure to the tumor extract allows these dendritic cells to become “teacher” cells for the rest of the immune system. These teacher cells, injected back into the patient, make up the vaccine. Once in the body, these newly educated dendritic cells recruit other immune cells to mount an attack against the tumor. Cytotoxic T Lymphocytes (CTL) are among the most effective immune cells recruited by the dendritic cells for attack. The educated and attacking CTLs can now be isolated from the blood, expanded in number in the laboratory, and then re-injected into the patient. This process is known as adaptive T-cell transfer.
University of Pennsylvania’s Ovarian Cancer Research Center combined a dendritic cell vaccine (OC-DC) approach with adaptive T-cell transfer to treat women with recurrent ovarian cancer. In results from the ongoing phase I trial (NCT01312376) that were presented at the American Association for Cancer Research conference in April, 2013, the tumor vaccine was given to 31 women and 19 showed a clinical benefit (61%), with 9 showing no evidence of disease and continued on maintenance with the vaccine. The combination of both the vaccine therapy with the adaptive T-cell transfer showed a 75 percent clinical benefit.
Immunotherapy approaches are experimental, but early clinical trial data suggest the potential for real benefit. Immunotherapy by its nature is highly personalized. A Clearity patient has the opportunity to find a trial that fits her personal molecular profile and potentially leverages her very unique immune system.