by Kalie VanDewater
Reporting of race and ethnicity data and representation of different racial and ethnic populations in OB/GYN clinical trials are subpar, although improvement is possible, according to findings published in JAMA Surgery.
“Although trial results inform policies affecting health for the entire U.S. population, trials often feature nonrepresentative cohorts,” Jecca R. Steinberg, MD, MSc, a third-year resident at Northwestern University Feinberg School of Medicine, and colleagues wrote. “Skewed participant demographics create a racial data gap; the individuals most affected by health disparities may be inadequately considered in solutions. When research systematically overlooks marginalized populations, it fails to account for the impact of social determinants of health that often fall along racial lines in the U.S. due to the role of structural and interpersonal racism.”
Steinberg and colleagues cross-sectionally analyzed OB/GYN studies registered on ClinicalTrials.gov from Oct. 1, 2007, to March 9, 2020, and linked them to publications on PubMed published before October 2021. Trials that were completed but did not have an associated publication were reviewed manually.
The researchers followed U.S. Census and HHS guidelines to evaluate the primary outcomes: the reporting of race and ethnicity data and representation of races and ethnicities, which was defined as the proportion of participants in each trial that belonged to a certain racial or ethnic group. Hispanic/Latina was categorized as both a race and ethnicity.
The researchers also identified the specialty and subspecialty and funding information for each trial.
In total, 1,287 trials with 591,196 participants were registered on ClinicalTrials.gov and 1,147 trials with 821,111 participants were publications of clinical trials.
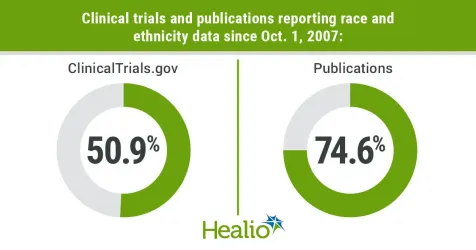
Race and ethnicity reporting
Most trials (50.9%) and publications (74.6%) reported race and ethnicity. Among trials reporting race and ethnicity, the most reported races were white (95.6%) and Black (92.7%), followed by Asian (82.9%), American Indian or Alaskan Native (69.5%) and Hispanic/Latina (62.6%).
Subspecialty analyses showed that reproductive endocrinology and infertility trials had the lowest odds of reporting race and ethnicity compared with obstetrics trials, which reported these data most. On ClinicalTrials.gov, the proportions of these studies reporting race and ethnicity were 41.9% for reproductive endocrinology and infertility trials and 54.2% for obstetrics trials; among publications, the percentages were 39.6% and 79.6%, respectively.
By specialty, gynecology trials were less likely to report race and ethnicity vs. obstetrics trials (adjusted OR = 0.54; 95% CI, 0.38-0.75).
There were no significant differences in reporting when analyzing funding source type.
Race and ethnicity representation
Notably, all trials and publications underrepresented American Indian or Alaskan Native, Asian, Black and Hispanic/Latina groups in their cohorts compared with U.S. Census estimates. However, the most diverse cohorts were in obstetrics and family planning trials.
Among subspecialties, Black representation was lowest in gynecologic oncology compared with obstetrics trials (ClinicalTrials.gov aOR = 0.04; 95% CI, 0.02-0.09; publications aOR = 0.06; 95% CI, 0.03-0.11). Urogynecology and reproductive endocrinology and infertility also had low representation of Black people.
Similar to Black representation, Hispanic/Latina representation was also lowest in gynecologic oncology vs. obstetrics trials (ClinicalTrials.gov aOR = 0.05; 95% CI, 0.02-0.14; publications aOR = 0.23; 95% CI, 0.1-0.48), followed urogynecology, reproductive endocrinology and infertility and other benign gynecology.
Academic and government-funded trials enrolled more American Indian or Alaskan Native, Asian, Black and Hispanic/Latina participants compared with industry trials (ClinicalTrials.gov, 29.4% vs. 15.1%; publications, 40.9% vs. 23.7%).
“In the setting of escalating racial and ethnic health inequities, the OB/GYN community must develop mechanisms for monitoring and improving inclusion of underrepresented groups in clinical trials,” Steinberg and colleagues wrote. “Stakeholders must prioritize transparency, accountability, diversity and equity to transform research infrastructure and adequately serve marginalized communities. Until we mend the racial disparities of clinical trials, research will continue contributing to unjust health outcomes borne by these communities.”
This article was published by Healio.


