The Basics
Most patients with advanced disease (diagnosed at stage III or IV) have no visible disease remaining after completing surgery and chemotherapy that includes platinum (usually carboplatin or cisplatin). Unfortunately, despite initial response to treatment, many will see their disease recur.
Recurrence happens because chemotherapy may not eliminate all of the cancer cells (they are too small to be visible on a scan). Those cancer cells can continue to divide and ultimately form new tumors, which can happen months or years after treatment.
Therapeutic choices after recurrence are complex. Many factors, such as how much time has passed since treatment with platinum and which drugs were given after that, come into play.
Platinum status is important since it predicts the likelihood that re-treatment with platinum will be effective and influences choice of subsequent treatment.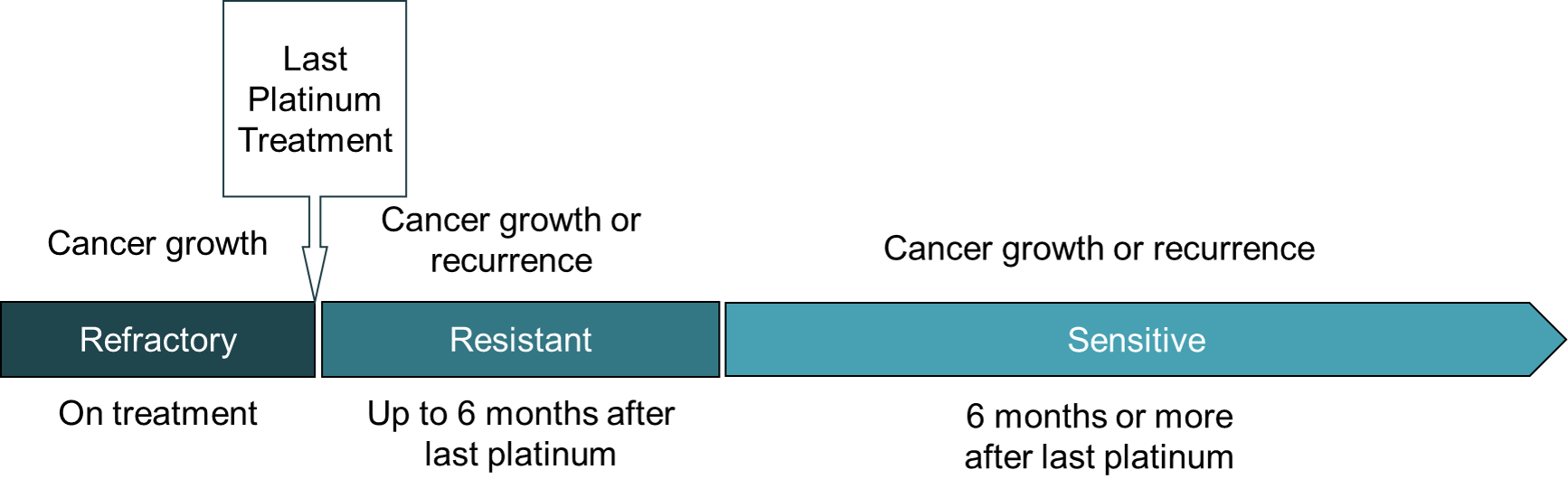
- A platinum-sensitive recurrence occurs six months or more after ending treatment with platinum. Re-treatment with a combination of platinum and another drug is often beneficial.
- A platinum-resistant recurrence occurs less than six months after the last platinum treatment. Non-platinum drugs are prescribed to treat it.
- Platinum refractory cancer continues to grow while on treatment or recurs within a month after the last platinum treatment. Non-platinum drugs are prescribed to treat it.
In addition to standard chemotherapy, there are new drugs in various stages of clinical development that may also be effective. For some of these drugs, there are published reports describing their side effects and effectiveness.
Some clinical trials enroll patients whose tumors have specific biomarkers or gene changes. Therefore, having results from tumor biomarker testing is essential for identifying such treatment options.
Treatments for Platinum-Sensitive Recurrence
 The tables below (click + to open) list the drugs most commonly used to treat cancer that has come back more than six months after the last platinum treatment. Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
The tables below (click + to open) list the drugs most commonly used to treat cancer that has come back more than six months after the last platinum treatment. Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
Standard of Care Treatments
To see how effective this drug is, click here, and scroll down.
To see side effects for this drug, click here.
To see prescribing information, click here.
| Drug(s) |
Clinical Notes |
| Carboplatin or Cisplatin |
Standard of care is Carboplatin or Cisplatin combined with Gemzar, Doxil or Taxol.
Adding Avastin to platinum-based chemo (and continuing as maintenance) can increase the time before cancer returns or gets worse.
To see how effective these drugs are, click here.
To see side effects for these drugs, click here.
To see prescribing information, click here. |
Carboplatin or Cisplatin
Gemcitabine (Gemzar)
Bevacizumab (Avastin) |
Carboplatin or Cisplatin
Paclitaxel (Taxol)
Bevacizumab (Avastin) |
Carboplatin or Cisplatin
Liposomal doxorubicin (Doxil)
Bevacizumab (Avastin) |
Additional Options for Low Grade Serous Cancer Patients
| Drug(s) |
Clinical Notes |
Trametinib (Mekinist)
|
MEK inhibitor (Mekinist) may be more effective than chemotherapy
To see detailed Phase III results, click here.
To see side effects associated with this drug, click here. |
|
Additional Options for Patients with Specific Biomarker Test Results
| Drug(s) |
Clinical Notes |
Dostarlimab (Jemperli)
Pembrolizumab (Keytruda) |
Treatment with an anti-PD-1 immune checkpoint inhibitor is approved for people whose tumor has dMMR* (dostarlimab or pembrolizumab), MSI-H** (pembrolizumab) or high TMB*** (pembrolizumab).
To see side effects associated with these drug, click here.
To see prescribing information, click here for dostarlimab or click her for pembrolizumab. |
Entrectinib (Rozlytrek)
Larotrectinib (Vitrakvi)
Repotrectinib (Augtyro) |
Entrectinib, Larotrectinib and Repotrectinib are approved for people with NTRK-fusion positive tumors
To see side effects associated with these drug, click here.
To see prescribing information, click here for entrectinib or click here for larotrectinib. |
| Selpercatinib (Revtevmo) |
Selpercatinib is approved for people with RET-fusion positive tumors
To see side effects associated with this drug, click here.
To see prescribing information, click here. |
Dabrafinib (Tafinlar)
Trametinib (Mekinist) |
The combination of Dabrafenib and Trametinib is approved for people with a BRAF V600E positive tumor
To see side effects associated with these drug, click here.
To see prescribing information, click here for dabrafenib or click here for trametinib. |
| *dMMR: Tumors with deficient Mismatch Repair
**MSI-H: Tumors that are microsatellite instability-high
***high TMB: Tumors with Tumor Mutational Burden ≥ 10 mutations/megabase |
Clinical Trials in Late Stage Development for Low Grade Serous Cancer*
| *For criteria used to highlight these trials, click here. |
| Drug(s) |
Trial Phase |
Clinical Notes |
| RAMP 301:
Avutometinib
Defactinib |
III
|
The combination of Avutometinib (VS-6766) and defactinib may have clinical activity, including in those who have previously been treated with a MEK inhibitor
To see detailed Phase II results, click here.
For ongoing trials with these or similar drugs, click here. |
There are drugs in earlier stages of development that already have results. Visit the Trial Results page to see those.
There are other drugs, including PARP inhibitor combinations and immunotherapies, being evaluated in clinical trials for treatment of platinum-sensitive recurrent ovarian cancer. Visit the clinical trials page and scroll down to see them.
Treatments for Platinum-Resistant or Refractory Recurrence
 The tables below (click + to open) list the drugs most commonly used for treating cancer has progressed on treatment (ie., refractory) or has come back less than 6 months after the last platinum treatment (ie., resistant). Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
The tables below (click + to open) list the drugs most commonly used for treating cancer has progressed on treatment (ie., refractory) or has come back less than 6 months after the last platinum treatment (ie., resistant). Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
Standard of Care Treatments
| Drug(s) |
Clinical Notes |
Paclitaxel (Taxol)
Bevacizumab (Avastin) |
Standard of care is treatment with single agent Taxol, Doxil or Topotecan.
Adding Avastin can increase the time before the cancer returns or gets worse.
To see results from the AURELIA trial, click here.
To see side effects for these drugs, click here.
To see prescribing information, click here. |
Liposomal Doxorubicin (Doxil)
Bevacizumab (Avastin) |
Topotecan (Hycamtin)
Bevacizumab (Avastin) |
| Mirvetuximab Soravtansine (Elahere) |
For people whose tumor has high levels of Folate Receptor alpha, Mirvetuximab Soravtansine may be as effective as and have more manageable side effects than standard chemo
To see results from the MIRASOL trial, click here.
To see side effects for this drug, click here.
To see prescribing information, click here. |
Trastuzumab Deruxtecan
(Enhertu) |
For people whose tumor is HER2-positive (IHC3+), Trastuzumab Deruxtecan may provide clinical benefit
To see results from the DESTINY-PanTumor02 trial, click here.
To see side effects for this drug, click here.
To see prescribing information, click here. |
Additional Options for Low Grade Serous Cancer Patients
| Drug(s) |
Clinical Notes |
Trametinib (Mekinist)
|
MEK inhibitor (Mekinist) may be more effective than chemotherapy
To see detailed Phase III results, click here.
To see side effects associated with this drug, click here. |
|
Additional Options for Patients with Specific Biomarker Test Results
| Drug(s) |
Clinical Notes |
Dostarlimab (Jemperli)
Pembrolizumab (Keytruda) |
Treatment with an anti-PD-1 immune checkpoint inhibitor is approved for people whose tumor has dMMR* (dostarlimab or pembrolizumab), MSI-H** (pembrolizumab) or high TMB*** (pembrolizumab).
To see side effects associated with these drug, click here.
To see prescribing information, click here for dostarlimab or click her for pembrolizumab. |
Entrectinib (Rozlytrek)
Larotrectinib (Vitrakvi)
Repotrectinib (Augtyro) |
Entrectinib, Larotrectinib and Repotrectinib are approved for people with NTRK-fusion positive tumors
To see side effects associated with these drug, click here.
To see prescribing information, click here for entrectinib or click here for larotrectinib. |
| Selpercatinib (Revtevmo) |
Selpercatinib is approved for people with RET-fusion positive tumors
To see side effects associated with this drug, click here.
To see prescribing information, click here. |
Dabrafinib (Tafinlar)
Trametinib (Mekinist) |
The combination of Dabrafenib and Trametinib is approved for people with a BRAF V600E positive tumor
To see side effects associated with these drug, click here.
To see prescribing information, click here for dabrafenib or click here for trametinib. |
| *dMMR: Tumors with deficient Mismatch Repair
**MSI-H: Tumors that are microsatellite instability-high
***high TMB: Tumors with Tumor Mutational Burden ≥ 10 mutations/megabase |
Clinical Trials in Late Stage Development*
| *For criteria used to highlight these trials, click here.
**BRCA-mutated patients have a mutation in the BRCA1 or BRCA2 gene detected in their blood (germline or inherited mutation) or tumor (somatic mutation) |
| Drug(s) |
Trial Phase |
Clinical Notes |
| OnPrime:
Olvimulogene nanivacirepvec
(Olvi-Vec)
Carboplatin
Paclitaxel (Taxol)
Bevacizumab (Avastin) |
III
|
Intraperitoneal infusion of an oncolytic virus (Olvi-Vec) may
increase the efficacy of a platinum triplet
To see detailed Phase II results, click here.
For ongoing trials with these or similar drugs, click here. |
| REFRαME-O1:
Luveltamab Tazevibulin |
II/III
|
For people whose tumor have some expression of Folate Receptor alpha, Luveltamab Tazevibulin may be more effective than standard chemotherapy
To see detailed Phase I results, click here.
For ongoing trials with these or similar drugs, click here. |
Clinical Trials in Late Stage Development for Low Grade Serous Cancer*
| *For criteria used to highlight these trials, click here. |
| Drug(s) |
Trial Phase |
Clinical Notes |
| RAMP 301:
Avutometinib
Defactinib |
III
|
The combination of Avutometinib (VS-6766) and defactinib may have clinical activity, including in those who have previously been treated with a MEK inhibitor
To see detailed Phase II results, click here.
For ongoing trials with these or similar drugs, click here. |
There are drugs in earlier stages of development that already have results. Visit the Trial Results page to see those.
There are other drugs being evaluated in clinical trials for treatment of platinum-resistant recurrent ovarian cancer. Visit the clinical trials page and scroll down to see them.

 The tables below (click + to open) list the drugs most commonly used to treat cancer that has come back more than six months after the last platinum treatment. Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
The tables below (click + to open) list the drugs most commonly used to treat cancer that has come back more than six months after the last platinum treatment. Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions. The tables below (click + to open) list the drugs most commonly used for treating cancer has progressed on treatment (ie., refractory) or has come back less than 6 months after the last platinum treatment (ie., resistant). Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
The tables below (click + to open) list the drugs most commonly used for treating cancer has progressed on treatment (ie., refractory) or has come back less than 6 months after the last platinum treatment (ie., resistant). Groups of drugs are used in combination. These drugs are listed in the National Comprehensive Cancer Network (NCCN) guidelines as preferred treatment options. Your doctor will know about these and other options that are also available. Please take this information to your doctor as an aid for your discussions.
